Atomic Shell
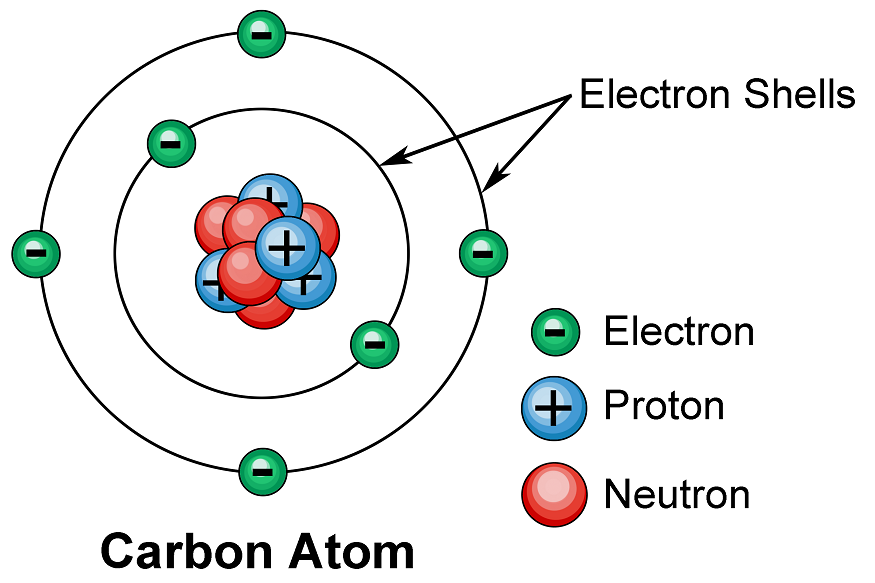
The term “atom” is derived from a Greek word that means “indivisible,” but we now know that atoms are made up of smaller parts known as subatomic particles. The electron, a tiny subatomic particle with a negative charge, was the first to be discovered. It is frequently represented as e–. Two larger particles were later discovered. The proton is a larger (but still tiny) subatomic particle with a positive charge, denoted by the symbol p+. The neutron is a subatomic particle that has approximately the same mass as a proton but no charge. It is denoted by either n or n0.
Atoms contain charged particles, and the number of charges in each atom varies by element. The positive charges are concentrated in a tiny nucleus, and electrons move around it in a space much, much larger than the nucleus.
What is an Atomic Shell Model?
To explain how electrons can have stable orbits around the nucleus, Bohr proposed his quantised shell model of the atom in 1913. The electrons’ motion in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; loss of energy causes electrons to spiral into the nucleus.
In an atom, electrons are arranged in shells surrounding the nucleus, with each successive shell moving further away from the nucleus. Electron shells are made up of one or more subshells, which are made up of one or more atomic orbitals. Electrons in the same subshell have the same energy as electrons in different shells or subshells.
The shells are sometimes designated with capital letters, beginning with K for the first shell, L for the second, M for the third, and so on. The maximum number of electrons that can occupy shells from one to seven would be 2, 8, 18, 32, 50, 72, 98, in that order. According to the general formula, the nth shell can theoretically hold up to 2n2 electrons.
What are Subshells?
Each electron shell is subdivided into one or more subshells, which are simply groups of one or more orbitals. Subshells are designated by the letters s, p, d, and f, with each letter representing a different shape.
For example, s subshells have a single spherical orbital, p subshells have three dumbbell-shaped orbitals at right angles to each other, and subshells d and f have more complex shapes with five and seven orbitals, respectively.
Difference between Shell, Subshell and Orbital
The terms shell, subshell, and orbital are used to describe the most likely paths that an electron can take. The main distinction between a shell, a subshell, and an orbital is that shells are made up of electrons with the same principal quantum number, subshells are made up of electrons with the same angular momentum quantum number, and orbitals are made up of electrons with the same energy level but different spins.
Thus, we can conclude that each atom has an electron shell labelled K, L, M, N, O, P, Q or 1, 2, 3, 4, 5, 6, 7, starting with the closest shell to the atomic nucleus and then moving outward. The average energy of electrons in outer shells is higher than that of electrons in inner shells.
Each shell consists of one or more subshells. Atomic orbitals make up each subshell.